Detection and Prevention of Mycobacterium avium subspecies paratuberculosis Infection in Dairy Cattle: A Review
By: Nicole Walters
Academic, 2017
Abstract
Johne’s disease, a chronic enteric infection of Mycobacterium avium subspecies paratuberculosis, is estimated to be present in over 90% of dairy cattle herds around the United States. No cure is known for the disease. Infection typically occurs during calfhood. Several management strategies have been shown to minimize the spread of disease. The first strategy is to remove the calf from an untested or test-positive mother within two hours of birth. Second, only feed heifer calves colostrum from test-negative mothers. Similarly, only feed heifer calves waste milk only from test-negative mothers. It is also common to remove test-positive animals from the herd, which is called the test-and-cull strategy. Several vaccines that have been shown to reduce the rate of infection are available, but they are not widely used because of the drawbacks associated with them. The vaccines that are currently commercially available are killed whole-cell vaccines. These show economic promise though they do not completely eradicate the disease. Alternatives to killed whole-cell vaccines, including subunit and live attenuated vaccines, are currently being researched. The majority of current research is on attenuated vaccines. Scientists are constantly looking for more effective vaccines in an attempt to halt the proliferation of this disease.
Introduction
Johne’s disease is a chronic enteric infection of Mycobacterium avium subspecies paratuberculosis (hereafter referred to as MAP) in ruminant animals [1,2]. Current findings indicate that the infection rate of MAP is increasing and an effective control program must be established [3]. Recent estimates suggest that at least 90% of dairy farms around the United States contain infected animals [4]. Estimates of economic losses caused by MAP infection range from $200 million to $1.5 billion annually [2,5]. Additionally, MAP infection has been linked to Crohn’s disease in humans [2]. However, no evidence has been found for a causal relationship between the two, merely an association [8].
Upon infection, the disease may progress silently in the intestinal tract of a ruminant for up to two years before symptoms appear [3]. During this time, the infection cannot be detected clinically or microbiologically. It is impossible to identify infected animals during this stage. After approximately two years, the infected animal begins shedding MAP, enabling other animals to be infected as well. The infection may still be sub-clinical at this time, meaning the host shows no clinical symptoms of infection. However, infection can be detected by ELISA and fecal culture at this stage. Finally, the clinical stage of infection begins as symptoms begin to arise. Symptoms of MAP infection include loss of milk production, weight loss, diarrhea, and death.
Interaction between MAP and the Host
MAP is an acid fast bacillus [5]. Acid fast bacilli have a protective layer of mycolic acid surrounding the peptidoglycan layer of the cell wall, which enhances their resistance to standard antibiotics [6]. MAP can be identified according to its need for mycobactin J siderphore to grow in artificial media [5]. The virulence of MAP is dependent on sigma factors, which are proteins required for the initiation of mRNA synthesis [4]. Virulent MAP also has the ability to evade host defense tactics [7].
Cattle are most susceptible to MAP infection as babies, though infection at a later age may be possible [1]. The susceptibility of 1 year old animals has been estimated to be 2.6% and that of 2 year old animals has been estimated to be 0.07% [1]. MAP can be introduced into the gut of a host through many pathways. The most common route of infection is fecal-oral. Infected feces in the area harbors live bacteria that can be taken up by the calf. Additionally, raw milk from an infected cow can cause infection. There is also a chance that a calf will be exposed to MAP in the environment. Lastly, MAP may be transmitted directly from the mother to the calf in utero.
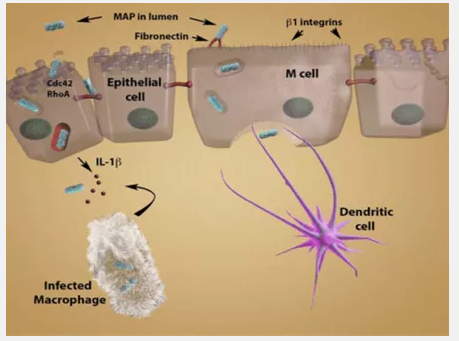
Once MAP is in the lumen, it infects the host as shown in Figure 1 – Route of Infection by MAP [12]. MAP is phagocytized by M cells in Peyer’s patches in the ilium, the third section of the small intestines [2,4]. MAP crosses the M cells to the basolateral side, where a macrophage phagocytizes the bacteria. At this point, the infected macrophage recognizes the presence of the pathogen and activates an immune response in an attempt to destroy the pathogen.
Figure 1 – Route of Infection by MAP
The immune system of the host can then take two pathways: the proper and improper immune responses. The proper immune response is to release pro-inflammatory cytokines IL-1B and TNF [2]. These elicit an early protective immune response, also called the TH1 response [2,8]. IFN-γ is then released from T-cells [2]. This activates antimicrobial mechanisms, and the pathogen inside the infected macrophage is destroyed. When this pathway is taken, the presence of MAP does not result in disease. However, MAP is often able to elicit an improper immune response [2,8]. The TH1 response is initially activated, but it may shift to a TH2 humoral response. The release of IL-10 blocks the TH1 response and the release of IL-13 induces the TH2 response [8]. The TH2 response suppresses T cell functions.
As the disease progresses, granulomas form in the ilium [3]. These granulomas (or lesions) are designed to isolate the infection, but they are not effective at eliminating infection in the case of MAP. These lesions reduce the uptake of available nutrients in the small intestine. The MAP feeds of the nutrients inside the small intestines and kills macrophages [5]. The infection also induces diarrhea, giving the animal less time to absorb nutrients from the food it intakes [3]. All of these effects result in less nutrients absorbed by the host. The host essentially starves though normal food consumption is maintained.
Once the host is infected, the disease progresses through three stages. First is the silent infection stage [3]. In this stage, the animal does not exhibit any clinical or microbiological signs of infection. The infection cannot be detected. This stage typically lasts around two years. The second stage is the sub-clinical infection stage. The animal still does not exhibit any clinical signs, but the infection may be detected by an ELISA or a fecal culture. At this point, the animal begins intermittently shedding viable MAP. The final stage of disease is the clinical infection stage. The animal now begins to show clinical signs of infection including weight loss, diarrhea, and death. At this stage, animals are typically shedding high levels of MAP.
Methods of Detecting MAP Infection
Three main methods of testing are used to detect MAP infection: the blood test, the milk test, and the fecal-culture test [9]. The blood test, also called the serum test, tests for MAP specific antibodies. Because vaccinated and infected animals produce similar antibodies, this test cannot distinguish between vaccinated and infected animals. An IDEXX Mycobacterium paratuberculosis ELISA is used to check for the presence of antibodies. An ELISA kit contains immobilized antibodies in a well plate [10]. The test serum is added to the plate and given time to interact. Targeted antibodies in the serum will bind to the immobilized antibodies. Then a secondary antibody with a marker is added and attaches to the targeted antibodies that bound. After washing, a substrate is added to activate the marker. If the marker appears, the targeted antibody is present in the serum. The blood test gives some false negatives [9]. An analysis of the reliability of the test showed it to be a moderately accurate test using the Area Under the Curve (AUC) method [11]. The second test, the milk test, also uses an ELISA. This test also gives some false negatives, but it is less likely to give positive results for vaccinated animals.
The third test is the fecal-culture test. A fecal sample from the animal in question undergoes a centrifugation and resuspension process to isolate the bacteria from the feces [8]. This sample is then incubated for 14 weeks on selective media and monitored weekly for growth [11]. Bacterial growth is observed for colony morphology, growth rate, mycobactin dependence, and acid fast staining to determine if it is MAP. This test only detects animals that shed MAP. For this reason, this test does not give false positive results for animals that are vaccinated. However, it also cannot detect animals in the earlier stages of infection.
Current Methods of Protecting Against MAP Infection
Currently, there are three main approaches to protecting against MAP infection: employing management strategies to reduce transmission, testing and culling, and vaccination [3]. Certain management strategies have been identified to reduce the risk of MAP infection. When a calf is born to an untested or test positive mother, the calf should be removed from the mother within 2 hours of birth to reduce the possibility of infection from the mother [11]. Heifer calves should only be fed colostrum from test negative mothers. Colostrum is the first milk a cow gives after parturition. Similarly, heifer calves should only be fed waste milk from test negative mothers [11]. The second approach to managing MAP infection is the test-and-cull method. In this method, all cattle are tested for MAP infection and any infected animal is sold.
Some killed whole-cell vaccinations are also currently available. These vaccines have not been shown to completely prevent infection [9]. They do reduce the rate of infection, slow progression of disease, and reduce shedding of MAP. However, it has been shown that infection rate can be similarly reduced by employing the aforementioned management changes [9]. Still, the vaccinations show economic promise. 80% of vaccinated calves tested positive for MAP specific antibodies by three to six months. However, this did not necessarily correlate with protection as the test cannot distinguish between immunity due to vaccination and immunity due to infection. Despite the potential to reduce infection, vaccines are not widely used in the United States due to the drawbacks of vaccination [5,9]. Currently three killed whole-cell vaccines are available worldwide, and one (Mycopar®) is approved for use in the United States [5]. One major concern of MAP vaccination is that it reduces the efficacy of Mycobacterium bovis testing [9]. The MAP antibodies produced by vaccinated animals cause the traditional M. bovis ELISA to test positive. M. bovis causes tuberculosis in cattle, so reduction in effective testing could cause an outbreak of tuberculosis. However, new ELISAs for M. bovis show potential for not interacting with MAP antibodies. An additional issue with MAP vaccinations is the formation of granulomas at the injection site [5]. Also, accidental injection of a human causes the formation of large granulomas.
Future Research
An ideal vaccine would prevent infection (protecting against horizontal transmission) as well as promote protective immunity (protecting against vertical transmission) [5]. An attractive vaccine would lower fecal shedding levels, lower tissue colonization, and lower clinical disease incidence. The vaccine must be pungent enough to evoke a TH1 protective immune response. It must also inhibit the shift to the TH2 humoral response to ensure MAP is destroyed upon infection.
Subunit vaccines are designed to overcome the drawbacks of whole-cell vaccines [5]. Whole-cell vaccines cause inflammation and granuloma formation at the injection site. A subunit vaccine would not produce this effect. Additionally, whole-cell vaccines interfere with tuberculosis and paratuberculosis diagnostic testing [3]. A subunit vaccine can specifically include MAP antigens that are not present in other Mycobacterium species. The antibodies produced for these antigens would not test positive on a tuberculosis assay. Subunit vaccines that are currently available do not offer complete protection [5]. Additionally, subunit vaccines are expensive to produce. An effective subunit vaccine must induce a strong TH1 response since IFN-γ, produced as a result of the TH1 response, is crucial to clearing an MAP infection early on [3]. Hsp70 is a promising candidate for a subunit vaccine. Once the antigen is identified, it can be delivered using a different type of bacteria as a host (e.g. Salmonella or Lactobacillus) [5].
Live attenuated vaccines are also being investigated [3]. Attenuated strains of MAP must adhere to the “Goldilocks rule,” which states that the extent of attenuation must be just right to induce an immune response without infecting the host [5]. Some advantages of using live attenuated strains of MAP for vaccines include ease of delivery, components that enhance immune response to MAP, and induction of a broad cellular immune response. Sigma factors are essential to the virulence of MAP, so they are promising candidates to producing live attenuated vaccines [4]. However, studies have shown that a single gene knockout may not be enough to reduce the virulence of MAP to a controllable level [5]. One sigma factor being researched as a target for gene deletion is the sigL gene. sigL is activated in the early stages of macrophage infection, which suggests it is an important virulent factor in MAP [4]. MAP that do not express sigL have a reduced ability to survive damages to the cell wall and oxidative stress. Additional candidates for gene deletion are Δre1A and ΔpknG [3]. Of the two, Δre1A is a more effective candidate.
Further research necessary in this field is centralized around creating more effective vaccinations [5]. A better understanding of how MAP evades hosts’ immune defenses would improve the ability to generate effective live attenuated vaccines [5,7]. The current pipeline for choosing an effective vaccine contains three phases [3,5]. The first phase is testing a macrophage culture for the expression of antibodies. The second phase is testing in a mouse model. Recently the validity of the second phase has been questioned [5]. Mice are not natural hosts for MAP, and MAP infection does not produce severe lesions in the small intestines of mice. Since this is a major factor in Johne’s disease, it is not surprising that data collected from mice trials does not correlate well to vaccine efficacy. The final stage is testing in a host [3,5]. This is the most appropriate test for the effectiveness of a vaccine, but it is done to limited candidates since it is expensive and time consuming [5]. Because the pipeline used to determine promising vaccine candidates is flawed, many vaccines are determined ineffective during testing.
Conclusion
Johne’s disease is a chronic enteric infection of MAP bacteria. Infection typically occurs at a young age and cannot be cured. Several management strategies are employed to reduce the risk of infection of calves including: (1) removing the calf from an untested or test-positive mother within two hours of birth, (2) feeding heifer calves colostrum only from test-negative mothers, and (3) feeding heifer calves waste milk only from test-negative mothers. A test-and-cull strategy is also often employed, where test-positive animals are removed from the herd. Vaccination against MAP infection is not a common practice due to the drawbacks of the vaccines, though it has been shown to reduce the rate of MAP infection. Current commercially available vaccines are killed whole-cell vaccines. These do not provide complete protection against infection, but are shown to have economic promise. Scientists are currently researching alternatives to killed whole-cell vaccines, including subunit vaccines and live attenuated vaccines. Live attenuated vaccines are being studied the most and show the most promise for an effective vaccine.
References
- Kirkeby, Carsten et al. “Simulating the Epidemiological and Economic Impact of Paratuberculosis Control Actions in Dairy Cattle.” Frontiers in Veterinary Science 3 (2016): 90. PMC. Web. 7 Dec. 2016.
- Casey, Maura E. et al. “Analysis of the Bovine Monocyte-Derived Macrophage Response to Mycobacterium Avium Subspecies Paratuberculosis Infection Using RNA-Seq.” Frontiers in Immunology 6 (2015): 23. PMC. Web. 6 Dec. 2016.
- Park, Hong-Tae, and Han Sang Yoo. “Development of Vaccines to Mycobacterium Avium Subsp. paratuberculosis Infection.” Clinical and Experimental Vaccine Research 5.2 (2016): 108–116. PMC. Web. 6 Dec. 2016.
- Ghosh, Pallab, Howard Steinberg, and Adel M. Talaat. “Virulence and Immunity Orchestrated by the Global Gene Regulator sigL in Mycobacterium Avium Subsp. Paratuberculosis.” Ed. A. Camilli. Infection and Immunity 82.7 (2014): 3066–3075. PMC. Web. 6 Dec. 2016.
- Bannantine, John P. et al. “A Rational Framework for Evaluating the next Generation of Vaccines against Mycobacterium Avium Subspecies paratuberculosis.” Frontiers in Cellular and Infection Microbiology 4 (2014): 126. PMC. Web. 6 Dec. 2016.
- Khan, Tarek Mahbub, PhD. “Bacterial Morphology I” Basic Medical Microbiology. 30 Dec. 2015. Web. 7 Dec. 2016.
- Facciuolo, Antonio et al. “Marked Differences in Mucosal Immune Responses Induced in Ileal versus Jejunal Peyer’s Patches to Mycobacterium AviumSubsp. paratuberculosis Secreted Proteins Following Targeted Enteric Infection in Young Calves.” Ed. Srinand Sreevatsan. PLoS ONE 11.7 (2016): e0158747. PMC. Web. 7 Dec. 2016.
- Shu, Dairu et al. “Diverse Cytokine Profile from Mesenteric Lymph Node Cells of Cull Cows Severely Affected with Johne’s Disease.” Clinical and Vaccine Immunology : CVI 18.9 (2011): 1467–1476. PMC. Web. 7 Dec. 2016.
- Tewari, Deepanker et al. “Mycobacterium Avium Subsp. Paratuberculosis Antibody Response, Fecal Shedding, and Antibody Cross-Reactivity to Mycobacterium Bovis in M. Avium Subsp. Paratuberculosis-Infected Cattle Herds Vaccinated against Johne’s Disease.” Ed. W. R. Waters. Clinical and Vaccine Immunology : CVI 21.5 (2014): 698–703. PMC. Web. 7 Dec. 2016.
- “ELISA- How Does the Test Work?” Biobest Laboratories Ltd. Web. 7 Dec. 2016.
- Chaffer, Marcelo et al. “Receiver Operating Characteristic-Based Assessment of a Serological Test Used to Detect Johne’s Disease in Israeli Dairy Herds.” Canadian Journal of Veterinary Research 72.1 (2008): 18–26. Print.
- Bannatine, John P. and Luiz E. Bermudez. “No Holes Barred: Invasion of the Intestinal Mucosa by Mycobacterium avium subsp. paratuberculosis.” Infection and Immunity. American Society for Microbiology. 12 Aug. 2013. Web. 7 Dec 2016.